NOBODY DOES PROTEINS LIKE WE DO
The biotech industry develops therapies specifically tailored for people and their conditions. Why should pets be any different?



The biotech industry develops therapies specifically tailored for people and their conditions. Why should pets be any different?
Using our Ambifect® Fc-fusion protein platform, Akston generates novel protein therapeutics that deliver customized, long-acting agents for a wide variety of diseases.

Therapeutic proteins that last longer for
obesity and cancer

Convenient, low-cost alternatives to monoclonal antibodies (mAbs) for chronic pain, dermatitis, and cancer
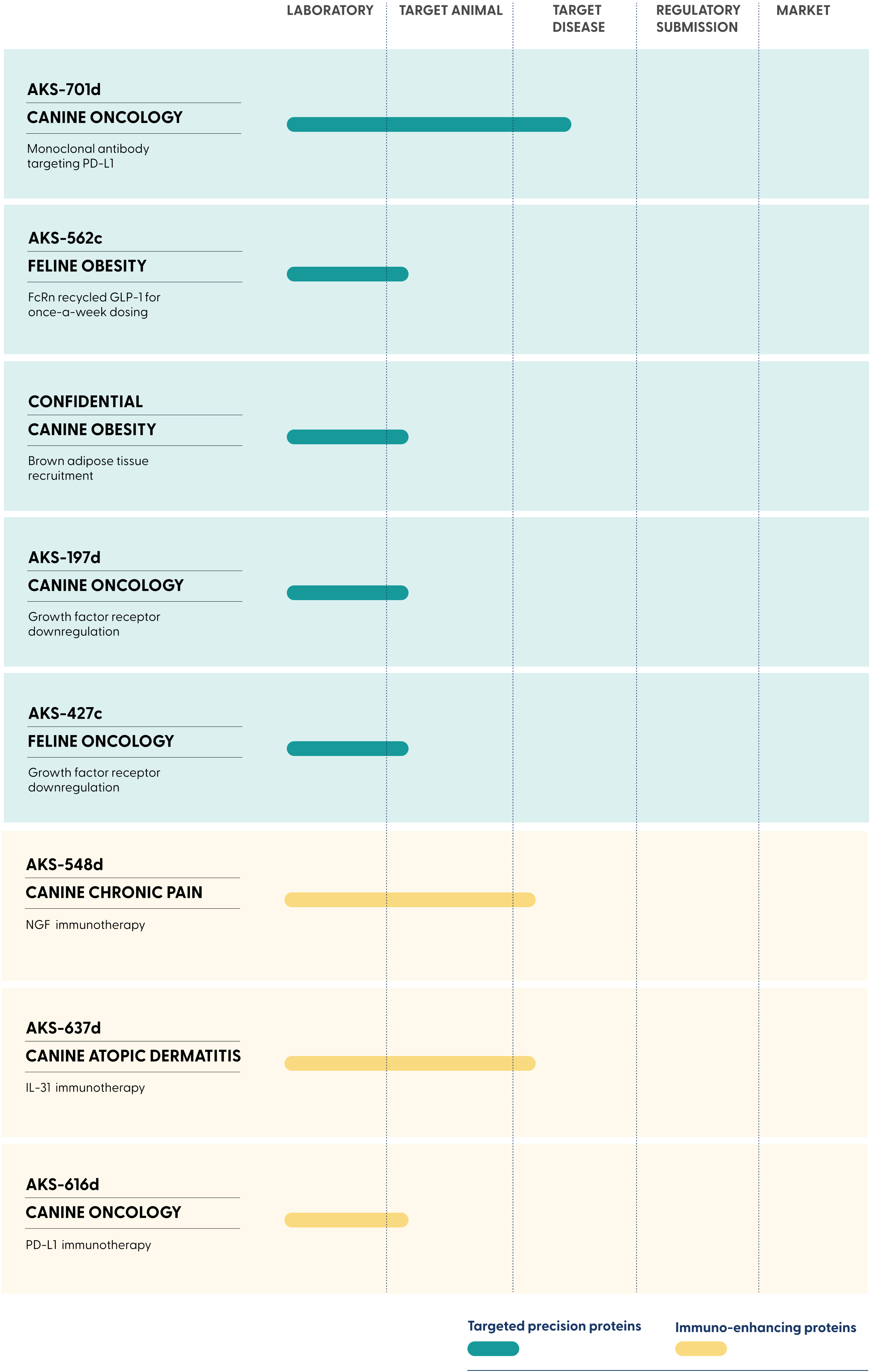
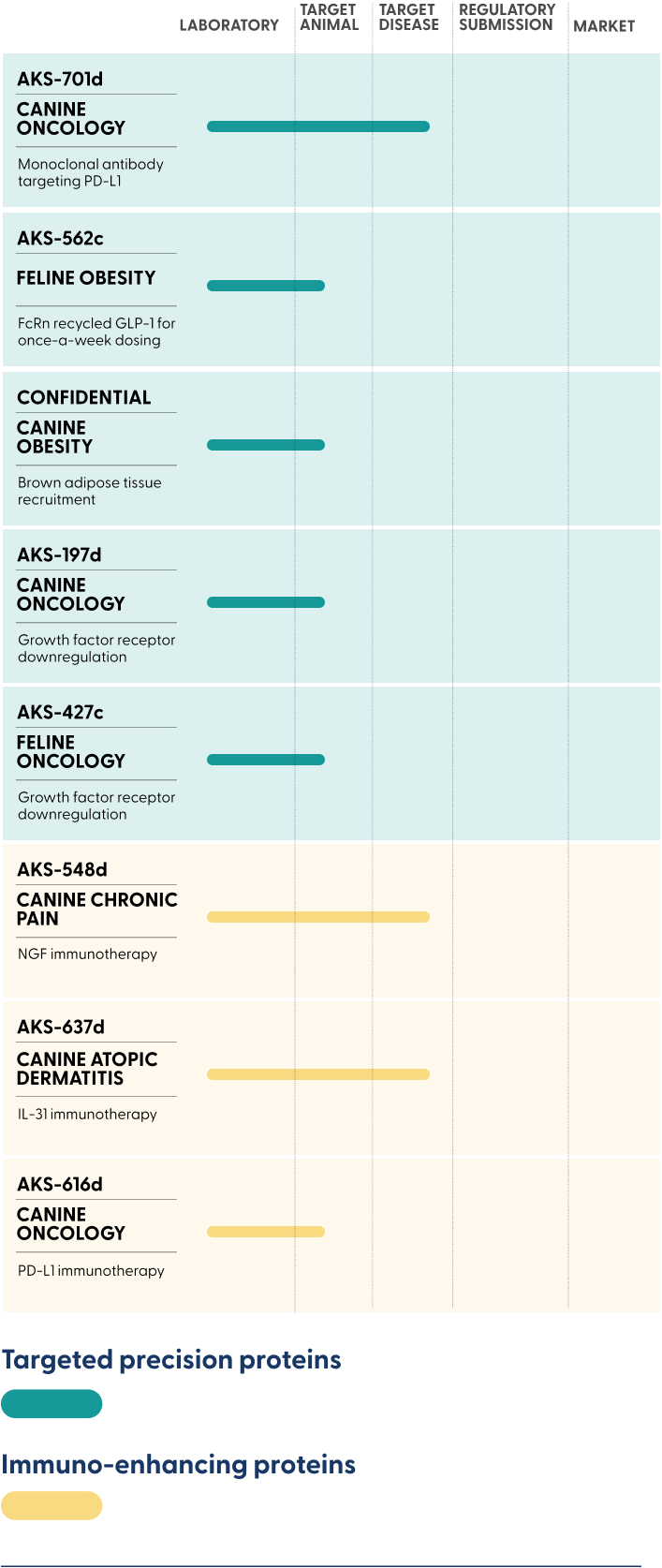
FcRn recycled GLP-1 for
once-a-week dosing
NGF immunotherapy



Get the latest Akston news tailored to your interests.